First drug to slow Alzheimer’s too costly for NHS
 Getty Images
Getty ImagesThe first drug to slow the progression of early stage Alzheimer’s won’t be available on the NHS in England because health assessment body NICE says the benefits “are too small to justify the costs”.
Lecanemab has been licensed by the UK medicines regulator, the MHRA, which means it can be prescribed privately.
In trials, the drug was shown to slow cognitive decline by about a quarter in patients in the early stages of Alzheimer’s over the course of 18 months.
But in draft guidance, NICE said there was a significant cost to the treatment including intensive monitoring for side-effects and fortnightly trips to hospital for patients.
Alzheimer’s Research UK said it was “a bittersweet moment”.
“The approval of lecanemab is a milestone moment but the decision by NICE not to approve it for the NHS is deeply disappointing,” said head of policy David Thomas.
Dr Samantha Roberts, chief executive of NICE, said the body had rigorously evaluated the available evidence, including the benefit for carers, but could only recommend treatments that “offer good value to the taxpayer.”
“Lecanemab provides on average four to six months’ slowing in the rate of progression from mild to moderate Alzheimer’s disease, but this is just not enough benefit to justify the additional cost to the NHS,” said Helen Knight, director of medicines evaluation at NICE.
Around 70,000 adults in England would have been eligible for treatment with lecanemab.
Wales and Northern Ireland often follow medical guidance in England.
The body which assesses newly-licensed medicines in Scotland has not yet made a decision on the value of the drug.
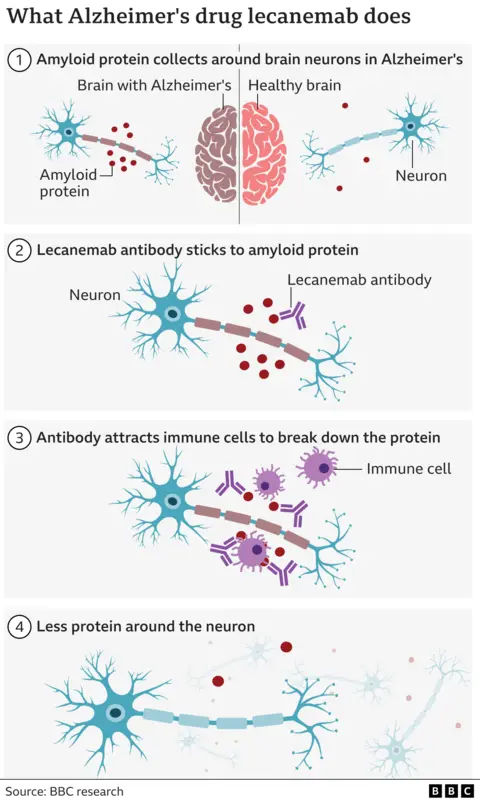
Lecanemab works by clearing a rogue protein called amyloid which builds up in the brains of Alzheimer’s patients. It is given as an intravenous infusion every two weeks.
Alzheimer’s researchers hailed the trial results as historic because no previous drug had convincingly shown that the underlying mechanism of the disease could be slowed.
But there were concerns over the frequent occurrence of what are known as amyloid-related imaging abnormalities (ARIA) which show up in MRI scans, such as small brain bleeds and temporary swelling.
Although most of these in trials were mild or without symptoms, in some cases participants required hospital treatment.
No price for the drug has been publicly announced in the UK but in the US it costs around £20,000 per patient per year.
Fiona Carragher of the Alzheimer’s Society told BBC News the decision would “lead to uncertainty and confusion for the nearly one million people living with dementia”.
Although the MHRA has approved the drug in the UK, it has set out some restrictions on patients who can receive the drug because of the risk of side effects.
People who carry two copies of the apolipoprotein E4 gene (ApoE4) will not be eligible, nor will those who are on blood thinners.
About 15% of people carry two copies of the APOE4 gene.
Lecanemab, whose brand name is Leqembi, has already been approved in the US, Japan and China.
But last month the European Medicines Agency (EMA) rejected a licence saying that the benefits were small and did not counterbalance the risk of serious side effects, especially bleeding and swelling in the brain.
In order to assess a patient’s eligibility for lecanemab they have to have the levels of amyloid in their brain measured. This is done either via a PET brain scan or by having a lumbar puncture.
Neither of these is a standard diagnostic tool for patients with suspected dementia and they are used only in research settings. It’s been estimated that only 2% of Alzheimer’s patients have access to one of these “gold standard” diagnostic methods.
The benefits of lecanemab are modest – indeed some argue they are barely noticeable. Then there are the potential side effects.
Yet many in the field regard this as a key moment, showing that Alzheimer’s is not unstoppable. Alzheimer’s is the most common form of dementia which is the leading cause of death in the UK.
The BBC’s Panorama followed patients on lecanemab and another new drug, donanemab.
In the programme, broadcast early this year, Prof Cath Mummery, consultant neurologist and head of clinical trials at the Dementia Research Centre, University College London, said although the benefits of the drugs were small, they represented a turning point.
“For the first time, we’ve got drugs that show that you can alter the course of Alzheimer’s disease, and that’s an extraordinary thing.”