NHS to offer ‘groundbreaking’ sickle cell gene therapy
 BBC
BBCA gene-editing therapy for sickle cell disease, with a price tag of £1.65m, is to be offered to patients on the NHS in England.
About 50 people a year with the inherited blood disorder are likely to receive it, experts say.
Prof Bola Owolabi, of NHS England, called it a “monumental step forward”, and said the one-off treatment Casgevy, also known as Exa-cel, “holds a very real prospect of a cure”.
A confidential agreement has been made with manufacturer Vertex on how much the NHS will pay.
Campaigners have described the treatment as “groundbreaking” and its availability on the NHS as a “milestone”.
Sickle cell disease can be life-threatening and cause recurring intense pain, when blood vessels become blocked by misshapen red cells.
About 15,000 people in England live with the condition, which mainly affects people of Black African and Black Caribbean heritage.
It is caused by genetic change that means people make haemoglobin – a key protein in red blood cells – that doesn’t work properly.
This results in red blood cells becoming sickle in shape and stiff and sticky – rather than flexible smooth discs.
These sickle cells do not live as long as healthy red blood cells and can clump as they travel around blood vessels – reducing oxygen to vital parts of the body.
This puts people at risk of organ damage, stroke, heart failure and a greatly reduced quality of life.
In trials all patients who received the therapy – which tweaks a specific gene and allows the body to make more healthy red blood cells – avoided stays in hospital for a year after treatment and most for three-and-a-half years. Further data is still being studied.
NHS chief executive Amanda Pritchard said the therapy “could be absolutely transformative – it could enable patients to live free from the fear of sickle cell crises hanging over them”.
Asiawu Imam, 26, lives in London, where she works as a nurse looking after people who have sickle cell disorder. She also lives with the condition.
When she was younger she was in and out of hospital three to four times a year with painful sickle cell crises.
“It feels like a stabbing pain, like someone is stabbing you form the inside outwards. It can last anything from half an hour to four days. It is excruciating,” she said.
The therapy being made available on the NHS gives her hope, and a sense to the community that people with the condition are being taken seriously.
“This is going to be a life-changing moment for many of my patients.”
How the gene therapy works
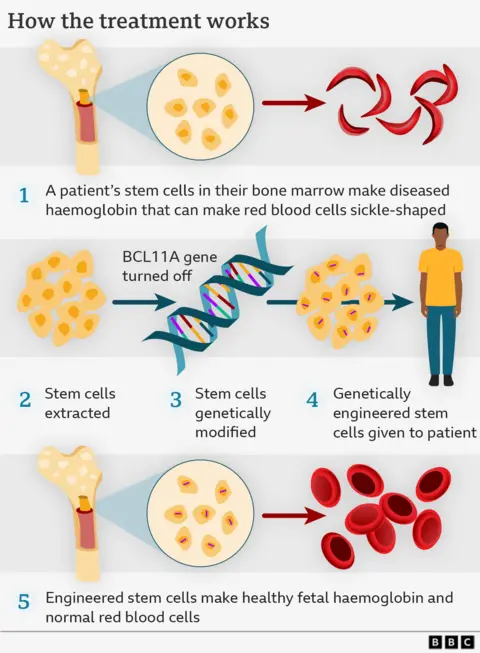
It is a multi-step process.
First, blood stem cells from a patient’s bone marrow (where all blood cells originate) are removed from the body.
In the laboratory, a gene-editing tool called Crispr is used.
This allows a specific gene to be pinpointed and very precise editing to take place.
However, instead of directly editing a faulty gene, Casgevy instead takes advantage of a process that happens when babies are in the womb, where they make red blood cells with foetal haemoglobin (a key protein that carries oxygen). This switches to the adult form once they are born.
Crucially foetal haemoglobin is not affected by sickle cell disease, so Crispr acts by dampening down the “switch” that makes the body produce the adult form.
Patients have to undergo “conditioning” chemotherapy to make sure their bodies are ready to accept the edited stem cells.
Modified stem cells are then transfused back into the body, where they multiply and increase the production of stable, well-functioning red cells.
The full treatment must be considered carefully – it can involve lengthy stays in hospital and may have side effects, including headaches and bleeding problems.
The only other current option for a cure is a stem cell transplant – but this can only happen if a closely matched donor is available. There is also a risk of the transplant being rejected.
The gene therapy will be available in specialist centres in London, Manchester and Birmingham to people aged 12 and over who get recurrent sickle cell crises and who cannot find a donor for a stem cell transplant.
John James, chief executive of the Sickle Cell Society, said: “We are absolutely thrilled to see this groundbreaking gene therapy treatment available on the NHS”, adding “the significance of this milestone for the sickle cell community could not be understated”.
He said the news would “give hope to many” and was “incredible”.
However, he added: “We remain acutely aware that not everyone with sickle cell will be eligible for the potentially life-changing benefits of Casgevy.
“There is still much work ahead to ensure that everyone living with sickle cell has access to the care, treatments, and support they deserve.”
The therapy has already been approved for another inherited blood disorder, transfusion-dependent beta thalassemia.
It is already being given to patients in other countries such as France, Germany and Italy.
Wales is also expected to provide it in the next few months.







